Purpose and Methods of Treatment of WasteWater
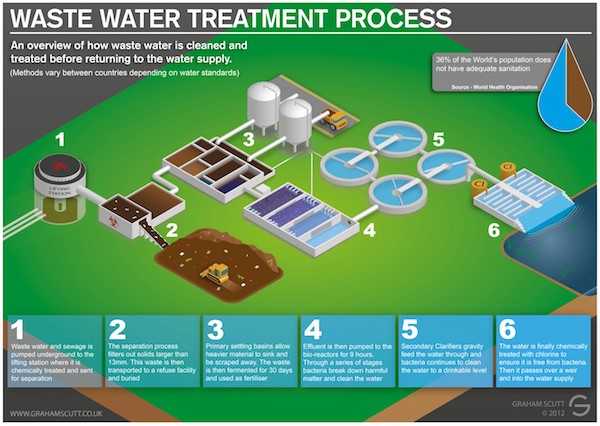
Wastewater treatment plays a crucial role in safeguarding public health and protecting the environment. As the world's population continues to grow and urbanize, the need for effective wastewater treatment methods becomes increasingly important. Wastewater contains a wide range of contaminants, including organic matter, nutrients, pathogens, and toxic substances, which must be removed before the water can be safely returned to the environment or reused.
Methods of WasteWater Treatment
There are different methods of water treatment the selection of which depends upon the type of source & purpose of use of water. Following are the methods of waste water treatment:
- Screening
- Plain sedimentation
- Coagulation & flocculation
- Secondary sedimentation
- Filtration
- Disinfection
- Aeration
- Softening etc.
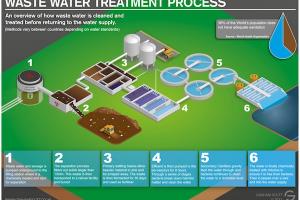
Screening:
Screening is the initial step in wastewater treatment, where large debris and solids are removed from the wastewater. It involves the use of screens or grates with different opening sizes to intercept and capture objects like sticks, rags, plastics, and larger particles. This prevents damage to downstream equipment and ensures smoother operation of subsequent treatment processes.
Plain Sedimentation:
Plain sedimentation, also known as primary sedimentation, is a process where wastewater is allowed to settle in large sedimentation tanks or basins. The flow velocity is reduced, allowing heavier solids and particles to settle to the bottom as primary sludge. The clarified effluent flows out from the top. This process removes settleable solids, including sand, grit, and organic matter, from the wastewater.
Coagulation and Flocculation:
Coagulation and flocculation are chemical processes used to remove fine suspended particles and colloidal matter from wastewater. Coagulation involves the addition of coagulants, such as aluminum sulfate (alum) or ferric chloride, to destabilize and neutralize the charged particles. Flocculation follows, where gentle mixing or agitation causes the formation of larger, aggregated particles called flocs. These flocs settle more readily during sedimentation or can be filtered more effectively.
Secondary Sedimentation:
Secondary sedimentation, also known as final clarification, is a process that follows biological treatment in the secondary stage. The wastewater, containing biological flocs from the activated sludge process, enters large settling tanks. The flocs settle under gravity and form secondary sludge, which is recycled back to the biological treatment process. The clarified effluent is collected from the top and moves to the next treatment stage.
Filtration:
Filtration is used to remove residual suspended particles and improve the clarity of the effluent. Various types of filters, such as rapid sand filters, dual media filters, or membrane filters, can be employed. These filters use porous media, such as sand or membranes, to physically strain out remaining solids as the wastewater passes through them.
Disinfection:
Disinfection is a critical step in water treatment that aims to eliminate or inactivate harmful microorganisms, such as bacteria, viruses, and parasites. Common disinfection methods include chlorination (using chlorine-based compounds), UV disinfection (using ultraviolet light), or ozonation (using ozone). These methods destroy or inactivate pathogens, making the water safe for consumption.
Aeration:
Aeration is used in biological treatment processes, such as activated sludge, to provide oxygen to microorganisms involved in the decomposition of organic matter. Aeration tanks or basins introduce air or oxygen into the wastewater, promoting the growth of aerobic bacteria. These bacteria utilize dissolved organic matter as a food source, converting it into carbon dioxide, water, and biomass.
Softening:
Water softening is a treatment method that targets the removal of hardness-causing minerals, such as calcium and magnesium ions, from the water. This is often achieved through processes like lime softening or ion exchange, where the hardness ions are replaced with sodium ions or removed through precipitation.
Treatment of Water
The prehistoric and the Old civilization established themselves near water sources. While the importance of water quantity for drinking purposes was apparent to our ancestors treatment processes were not well known. The methods of Waste Water treatment originally focused on improving the aesthetic and Sight qualities of drinking. Ancient Sanskrit & Ancient Greek writings recommended water treatment methods such as filtering through charcoal, exposing it to sunlight, boiling, & straining.
During the 1700's filtration was established as an effective means of removing the particles from the water and treating it. So the earlier purification process was based on filtering the water to remove the Impurities & appearance of colloidal particles present (EPA 2000).
During the mid to late 1800, scientists gained a greater understanding of the sources & effects of drinking water contaminants, especially those that were not visible to the naked eye. In the 1800's Louis Pasteur demonstrated the germ theory of disease, which explained how microorganisms could transmit disease through media like water. During the late nineteen & twentieth centuries, concerns regarding drinking water quality continued to focus mostly on disease-causing microbes known as pathogenic bacteria in public water supplies.
It was known that turbidity is not only an aesthetic problem, particles in source water such as fecal matter, could harbor pathogens. Therefore, filtration was established as an effective means of removing these particles. The clean & reliable water supply requirement was not recognized, until the second half of the nineteen century, when the nature of infectious disease was first recognized. The water supply becomes the major source to transmit these diseases. Therefore, proper treatment of water becomes essential. The process of making water suitable for certain use such as domestic, industrial, or any other use is called the treatment of water.
Also See: Hospital Waste
The degree & type of treatment depends on the source of water & the purpose for which the water is required. Generally, the water from surface sources is treated conventionally to make it suitable for domestic as well as industrial use. The groundwater is generally suitable for drinking purposes but should be treated for industrial use to remove the hardness of the water.
Purpose of Waste Water Treatment
It is desirable to treat water for a number of reasons including:
- To prevent pathogenic microorganisms from causing the disease.
- To control unpleasant taste & appearance of particles.
- To remove the excessive color of water & turbidity.
- To extract the chemicals & dissolved minerals.
Procedures for Waste Water Treatment
Despite the existence of a multitude of technological processes for the production of water for potable or other uses, the general principles of purification are approximately the same. These principles provide the following range of primary procedures:
- Removal from the water of heterophase contaminants by sedimentation or coagulation & sedimentation, filtration, &, less often, flotation. As a result of such treatment, water turbidity & color index are reduced.
- Elimination of admixture of active pathogenic bacteria & prevention of their reproduction (disinfection of water) by chlorination, iodination, Ozonation, silvering, electromagnetic radiation, & electrochemical & other methods.
- Adjustment of water composition in dissolved (homophase) admixtures. This stage can include a large diversity of technological processes depending on the composition & quality of the initial water. First, this includes the elimination of smell, taste. & toxic trace pollutants of water by methods of aeration & degassing, oxidation, adsorption, & removal from the water of iron, manganese, silicon, & fluorides.
- The final stage of water treatment might include water fluorination & softening (hardness removal). In regions with a deficiency of freshwater but the availability of sources of brackish & saline water, it may also be necessary to carry out water desalination.
- Special water treatment, for example by radiation method, as well its purification of specific contaminants including radioactive matters or particular highly toxic chemicals.
The processes & technologies used to remove the contaminants from water & to improve its quality are recognized all over the world. The choice of which treatment to use from a great variety of available processes depends on the characteristics of the water, the type of water quality problems to be present, & the costs of different treatments.
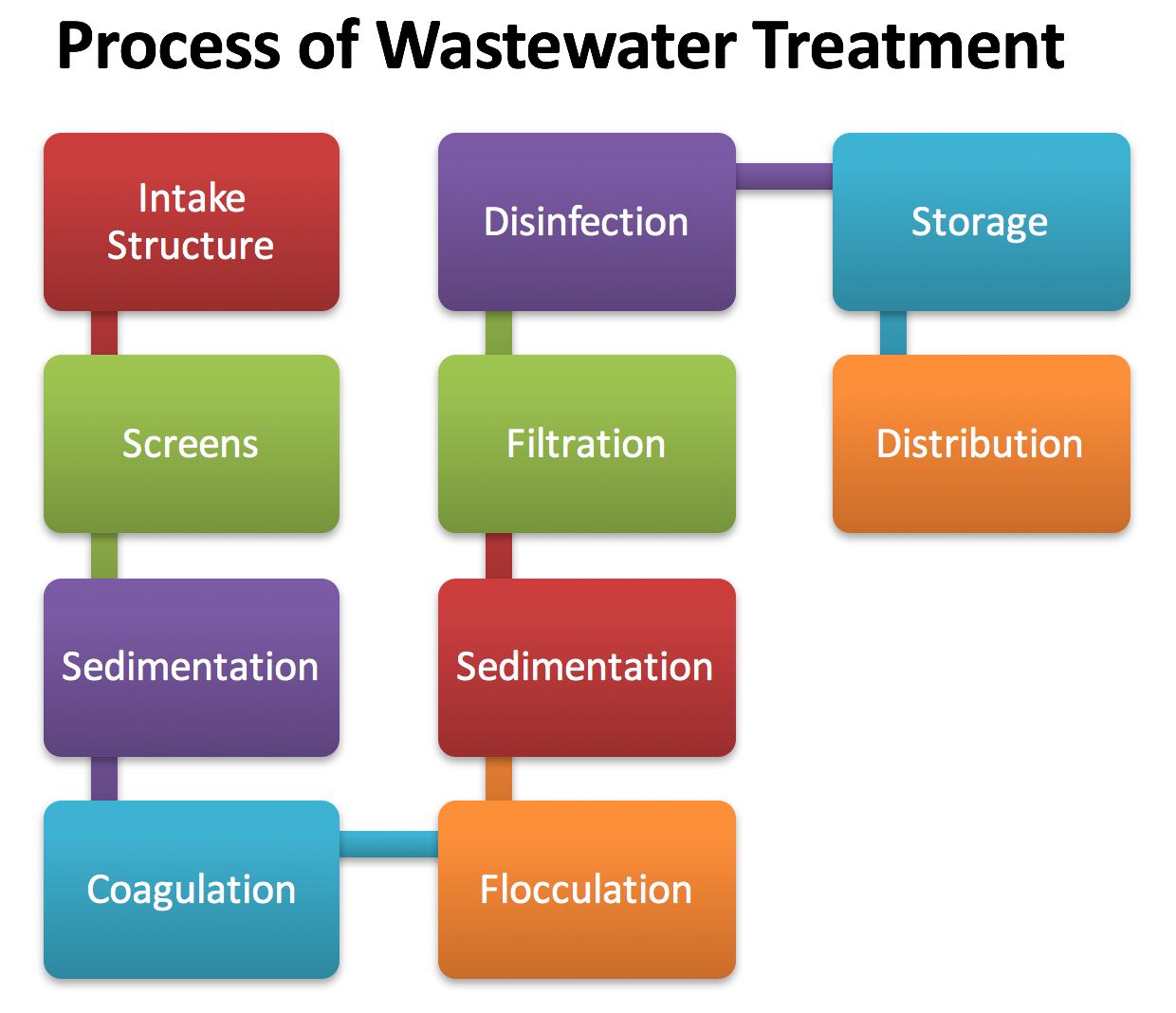
- As pointed out earlier that all the above techniques may or may not be used for treating the particular nature of water. The necessity of a few or all of these treatments depends solely upon the quality of available raw water. Before we describe these techniques in detail, let us summarize the functions served by each of these techniques in effecting the purification of raw water.
- Most of the big & visible objects, such as trees, branches, sticks, vegetation, fish, animal life, etc., present in raw water of surface material can be removed by bar & screen operations. This process is called screening. The coarser suspended material can then be removed by letting the water settle in sedimentation basins. The process is called plain sedimentation.
- The effectiveness of sedimentation may, however, be increased by mixing certain chemicals with water, to form flocculent precipitates, which carry the suspended particles as it settles. The process is called chemical coagulation. The finer particles in suspension, which may avoid settling in sedimentation basins even after using the chemical coagulation, may then be removed by filtering the water through filters. This process is called filtration
- Filtered water that may still contain pathogenic bacteria is then made bacteria-proof by adding certain chemicals such as chlorine etc. This process of killing germs is called disinfection.
- The resulting water, though now becomes safe, may not be attractive to the tongue of consumers. Unpleasant tastes & odors may then, therefore, have to be removed by adding certain chemical compounds such as activated carbon. This process is called adsorption.
- The resulting water may sometimes be much harder than the permissible level & may, therefore, have to be softened by a process called softening. Sometimes, the resulting water may be given further treatment, such as fluoridation (i.e. the addition of soluble fluoride for controlling dental caries), liming (i.e. addition of lime in order to control acidity & reduce the corrosive action), re-carbonation (i.e. addition of carbon dioxide so as to prevent deposition of calcium carbonate scale), the desalination (i.e. removal of excessive salt, if present)